Professor Khuloud T Al-Jamal

Head
Lo Shiu Kwan Kan Po Ling Professor in Pharmacy
Global STEM Scholar Professor
- BSc, PhD, FHEA, FRSC, FRPharmS
Biography
Professor Khuloud T Al-Jamal, Lo Shiu Kwan Kan Po Ling Professor in Pharmacy, is a Global STEM Professor, the Director of the Jockey Club STEM Lab of Advanced Therapies for Nanomedicines and The Head of Pharmacology & Pharmacy at LKS Faculty of Medicine, The University of Hong Kong. She served as The Head of Medicines Development at the Institute of Pharmaceutical Science, King’s College London (2020-2024) where she also developed herself as an independent researcher since her appointment as an Assistant Professor in 2011 to becoming a Full Professor in 2016. Professor Al-Jamal completed her PhD at the Centre for Drug Delivery Research and postdoctoral training at the Nanomedicine Lab, The School of Pharmacy, University of London (2000-2010). She undertook her pre-registration pharmacy training at University College London Hospitals. Her research focusses on developing nanomedicines and advanced therapies to improve treatment of neurological diseases and cancer. She published over 170 articles which attracted ~11,500 citations (by 2024). She supervised over 150 researchers and attracted over $HKD 200M of funding.
She is a recipient of the Royal Pharmaceutical Society of Great Britain Science Medal, becoming the third female to receive this award in a 43-year-history, Maplethorpe Fellowship for the promotion of pharmaceutical education and excellence in research (University of London), Biotechnology and Biological Sciences Research Council (BBSRC) New Investigator Award, the Controlled Release Society Nanomedicine and Nanoscale Delivery Young Investigator Award. She is a three-time winner of the Wellcome Trust Science Image Awards.
She is an editorial board member for several journals such as Journal of Controlled Release, Biomaterials Science, Scientific Reports, MedBioMed and Journal of Drug Targeting. She is a Fellow of Royal Society of Chemistry and The Royal Pharmaceutical Society of Great Britain.
Memberships & Editorships
Memberships:
- 2023 Crick University Partners Translational Fund (UK), Scientific Board Member
- 2023 iAward Sanofi Europe program, Scientific Board Member
- 2022- Brain Research UK, Scientific Board Member
- 2021 CRUK Early Detection & Diagnosis, Expert Review Panel Member
- 2021- Royal Society of Chemistry, Fellow
- 2022 MRC Nucleic Acid Therapy Accelerator Fund, Expert Panel Member
- 2020 NC3Rs CRACK IT, Review Panel Member
- 2019-2023 Children's Brain Tumour Drug Delivery Consortium, Steering Committee Member
- 2018-2024 London Centre for Nanotechnology, Steering Committee Member
- 2017-2019 COST Action CA17140 (EU)
- 2015- General Pharmaceutical Council (UK), Member
- 2013- Higher Education Academy, Fellow
- 2022- Royal Pharmaceutical Society of Great Britain, Fellow
Editorships:
- Biomaterial Science, Royal Society of Chemistry.
- Journal of Controlled Release, Elsevier.
- Scientific Reports, Nature Publishing Group.
- Journal of Drug Targeting, Informa.
- ChemMedChem, Wiley.
Honours and Awards
- 2024 Outstanding Medical Education Contribution and Championing an Inclusive, Fair and Diverse Faculty award, Education and People & Culture category nominee, KCL.
- 2023-24 Supervisory Excellence Award, KCL
- 2022-24 World’s Top 2% researchers, Stanford University analysis.
- 2022 Fellow of the Royal Pharmaceutical Society of Great Britain
- 2021 Fellow of Royal Society of Chemistry
- 2020 Teaching Excellence Award, KCL
- 2019 Controlled Release Society Young Investigator Award
- 2014, 2015 & 2016 The Wellcome Trust Science Image Award
- 2014 EPSRC Science Photo Award
- 2012 The Royal Pharmaceutical Society of Great Britain Science Medal
- 2005 Maplethorpe Research and Teaching Fellowship, The University of London
- 2001 GSK Graduate Fund Award for doctoral studies
- 2001 Overseas Research Scholarship Award, University of London
- 1998 Best Achievement Award in Undergraduate Studies, University of Petra.
Research Interests
Our lab is currently actively researching in the following 4 research topics:
- Brain Drug Delivery
- Drug delivery for brain diseases such as brain cancer and neurodegenerative diseases.
- Design and engineer nanoparticles that cross the BBB.
- Delivery of targeted diagnostic agents to detect brain disease lesions.
- Development of targeted nanomedicine-mediated cancer chemotherapy, RNAi therapy, CRISPR/Cas9 gene editing, cancer vaccines.
- Delivery of ‘theranostic’ (therapeutic and diagnostic) agents to the brain.
- Development of nanoparticles suitable for direct nose-to-brain delivery.
- Validation of therapeutic targets pre-clinically and translation to human.
- Cancer Delivery & Imaging
- Improved drug bioavailability
- Reduced off target toxicity
- Improved therapeutic window
- Anti-cancer drugs of poorly bioavailable drugs
- Nucleic acid such siRNA, mRNA and pDNA
- Targeted radiotherapy
- Immunotherapy
- Immune adjuvants
- Nucleic acid-based gene therapies
- Prophylactic and therapeutic vaccines
- Immunologically active drugs.
- Early Disease Detection
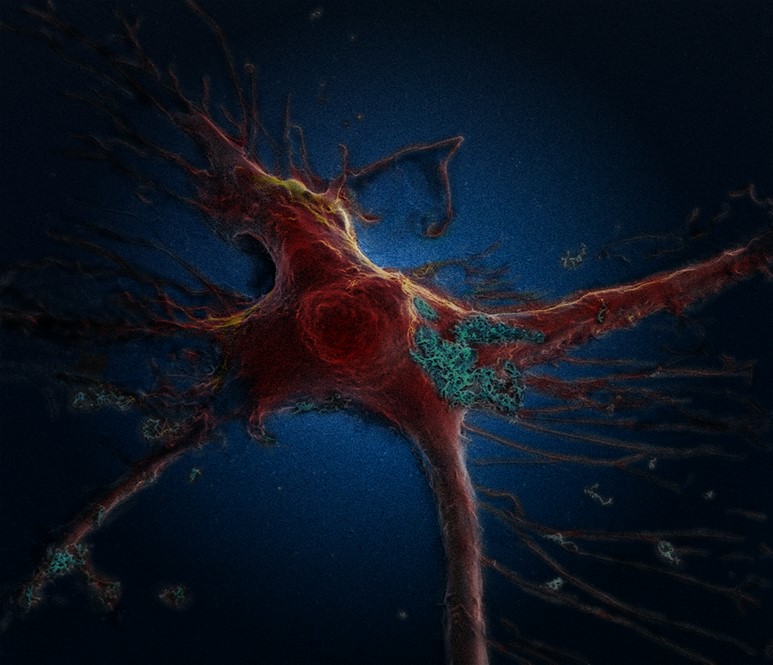 Brain astrocyte cell taking up carbon nano-needles, SEM
Khuloud T. Al-Jamal, Serene Tay & Michael Cicirko
Brain astrocyte cell taking up carbon nano-needles, SEM
Khuloud T. Al-Jamal, Serene Tay & Michael Cicirko
Brain diseases, such as brain cancers and central nervous system (CNS) disorders, are some of the most prevalent, devastating and yet poorly treated diseases. The presence of blood-brain barrier (BBB) and the immune privilege feature of the CNS remain the major cause of inefficient therapeutic outcomes.
Nanoparticles have a huge potential in the field of drug delivery into the brain because:
They are known to encapsulate more drugs
Nanoparticles can be formulated to achieve high loadings of drugs with different physiochemical properties (e.g. hydrophilic, hydrophobic, charged) to therapeutic levels.
They may improve the drug transport through the BBB when administered intravenously by intrinsic BBB crossing properties or targeting specific transport processes
Nanoparticles can be easily modulated in terms of physicochemical properties (e.g. size, charge, coating) and thus pass BBB through pathways such as transcytosis, endocytosis or combined mechanisms. They can also be engineered with ligands to target specific receptors.
They can facilitate non-invasive methods of delivery e.g. nose to brain
Nanoparticles can be designed to offer longer retention time in the nasal cavity e.g. in mucoadhensive matrix, provide protection of drug from degradation and promote drug transport by specific mechanisms.
We are interested in:
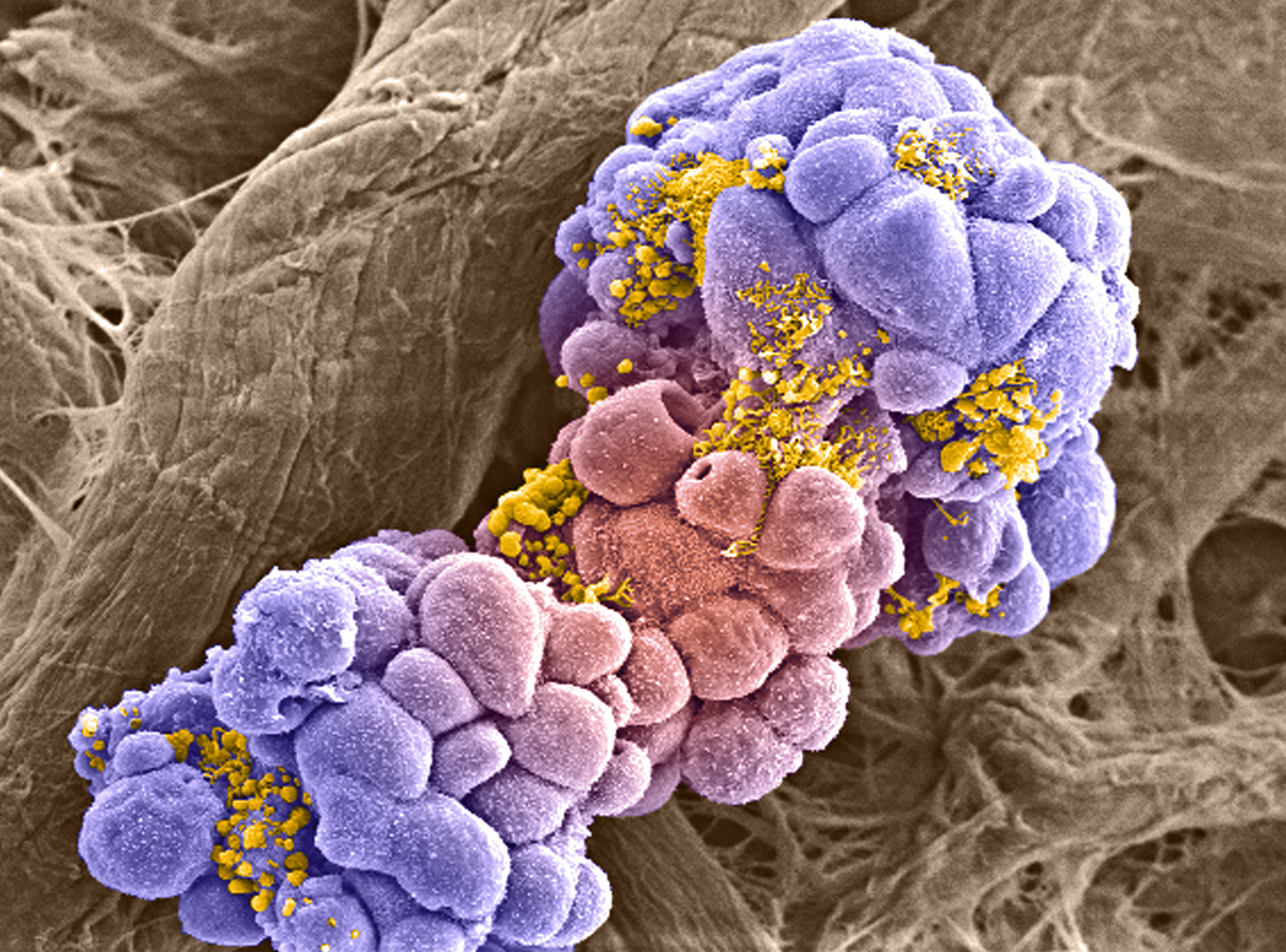 Breast cancer cell spheroid treated with anti-cancer drugs
Izzat Suffian & Khuloud T. Al-Jamal
Breast cancer cell spheroid treated with anti-cancer drugs
Izzat Suffian & Khuloud T. Al-Jamal
The use of nanotechnology in delivering chemotherapy, radiotherapy and gene therapy is a long-established approach with commercially available formulations available clinically. We are working on devising novel particulate formulations for either clinically approved or experimental drugs (chemical or biological drugs). Our aim is to improve efficacy through enhanced tumour targeting or improved pharmacokinetics. In addition to passive targeting, we use advanced techniques such as ligand or magnetic guided nanoparticles to concentrate drugs at the tumour site.
Nanoparticles have a huge potential in this arena as they can offer:
Our primary interests are the delivery of:
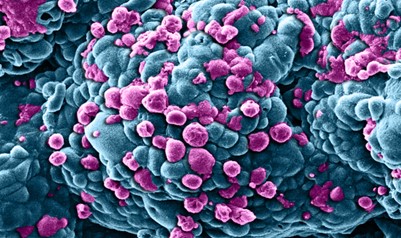 Breast cancer cells treated with nano sized drug carriers.
Khuloud T. Al-Jamal, David McCarthy & Izzat Suffian
Breast cancer cells treated with nano sized drug carriers.
Khuloud T. Al-Jamal, David McCarthy & Izzat Suffian
Immunotherapy is a revolutionary approach based on mobilising the patient’s immune system to eradicate tumours. Immunotherapy takes many forms including check point blockade, neoantigen vaccines and adoptive cell therapy. While there have been many successful immunotherapeutic drugs licenced, many only achieve clinical efficacy in a subset of patients. Furthermore, many of the therapies are associated with off target effects leading to toxicity. While great success has been achieved, we are working on developing nano-systems to both circumvent the short comings and improve the existing strategies.
Nanoparticles have a huge potential in this arena as they can offer targeted delivery of immunologically active molecules to the active site. Nanoparticles can be designed to concentrate drugs within tumours leading to greater local immune activation. Alternately, particles can be targeted to the cells of the immune system either in circulation or in lymphatic tissues. Nanoparticles can be formulated to achieve high loadings of drugs with different physiochemical properties (e.g. hydrophilic, hydrophobic, charged) at therapeutic levels.
Depending on a multitude of factors, such as size, shape composition etc., particles can selectively engage the immune system resulting in increased activation. Multiple agents can be included in a single nanoparticulate system to ensure a spacio-temporal relationship is established between synergistic components. Sequestering immunological agents within particles minimizes off target effects which may otherwise limit the therapeutic window.
We utilise a range of modalities, including polymeric, lipidic, and composite materials to deliver numerous small molecules and macromolecules either in isolation or in combination. Our primary interests are the delivery of:
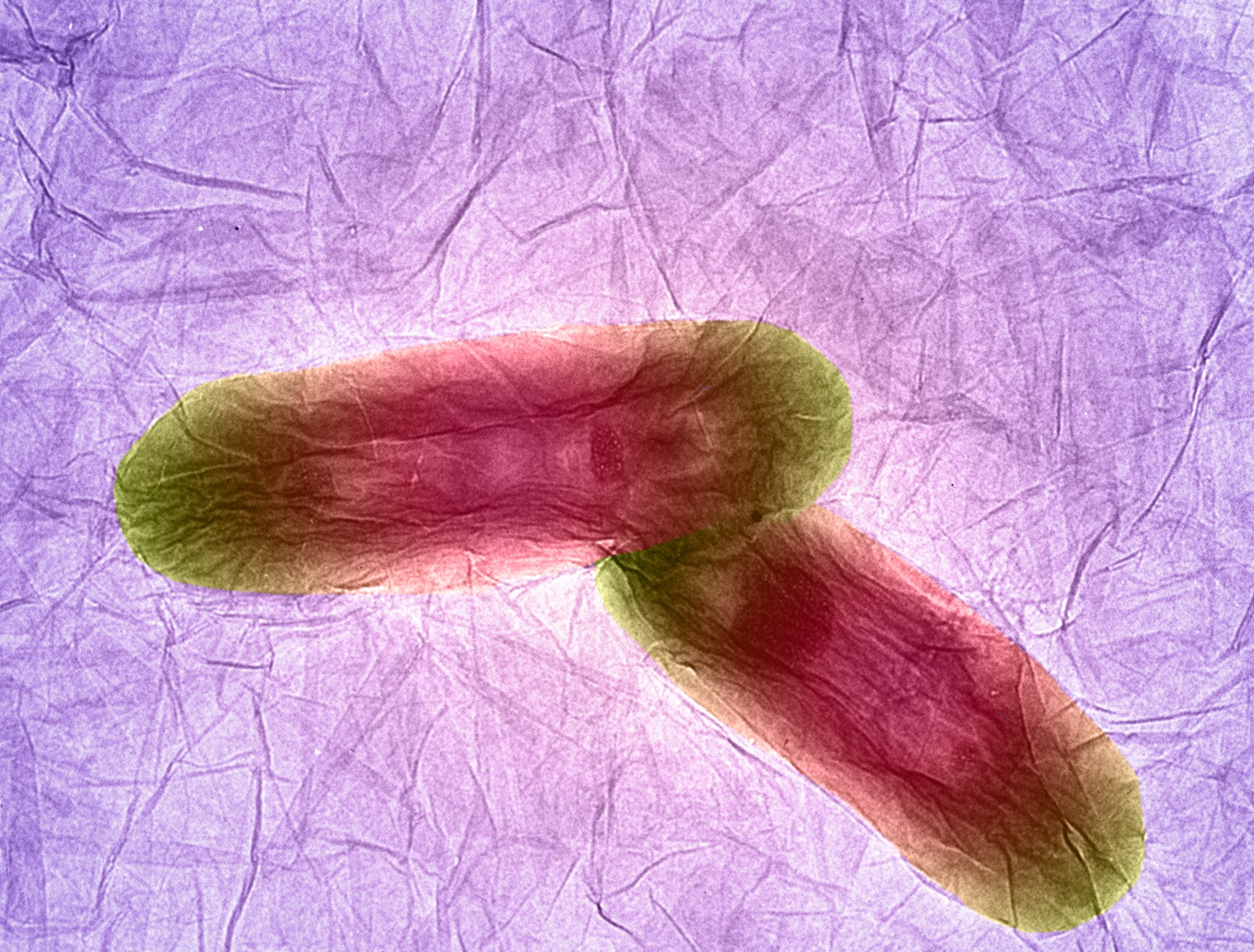 Bacteria sitting on graphene sheets, TEM
Izzat Suffian, Kuo-Ching Mei, Houmam Kafa & Khuloud Al-Jamal
Bacteria sitting on graphene sheets, TEM
Izzat Suffian, Kuo-Ching Mei, Houmam Kafa & Khuloud Al-Jamal
Certain variants of cancer such as pancreatic and ovarian cancer have a very high mortality rate, due to the disease being only detected at its later stages. Early detection of cancers in general has been correlated to a higher success rate of various treatment options and therefore a higher rate of patient survival, alongside reduced therapy duration and the associated side effects. Neurodegenerative diseases such as ALS and Parkinson’s disease would also benefit similarly from early detection in terms of managing motor dysfunction and dementia.
Cancer and neurodegenerative disease patients have been shown to have higher levels of certain circulating biomarkers (e.g. exosomes) compared to that of healthy individuals, of which their levels correlate with the stage of the disease. Detection of such circulating biomarkers therefore can be utilised as an early detection strategy for these diseases.
Nanotechnology can serve this field by improving the sensitivity of existing biosensing platforms.
Our team is interested in the following research topics: Development of ultra-sensitive assays for detection of circulating disease biomarkers e.g. exosomes in collaboration with engineers; identification and validation of novel circulating biomarkers for early disease detection and diagnosis in collaboration with clinicians; multiplex-based detection of disease biomarkers; translation of validated platforms as point-of-care (POC) diagnostic devices.
Publications
Publications Highlights
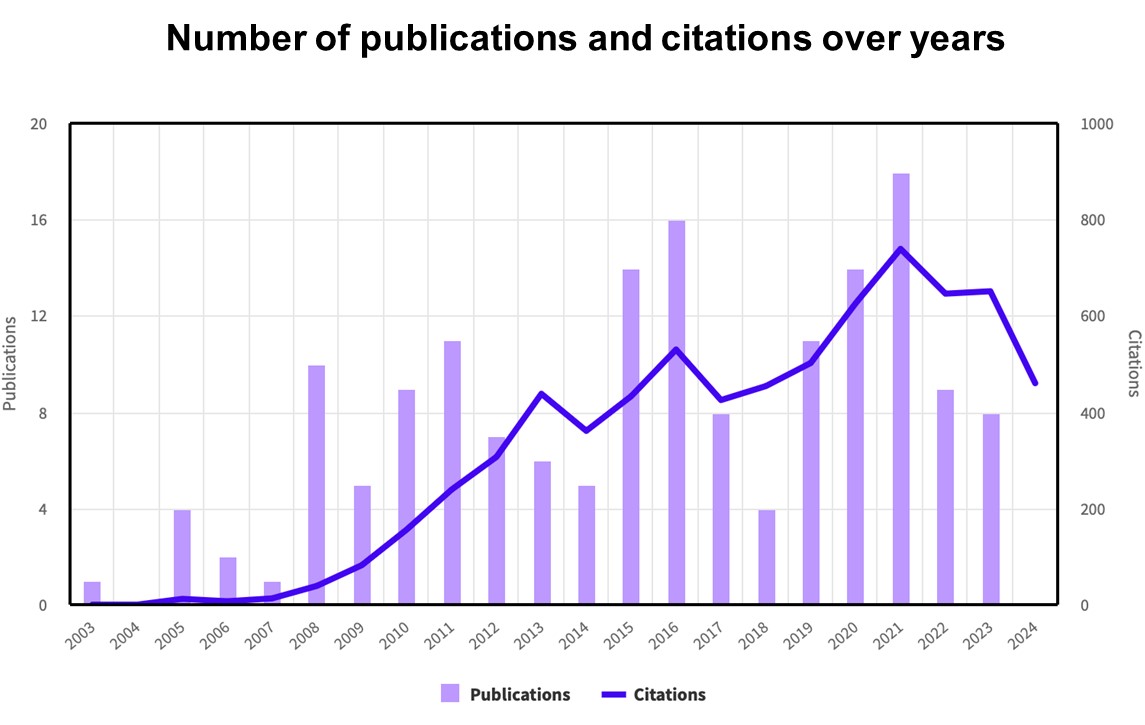
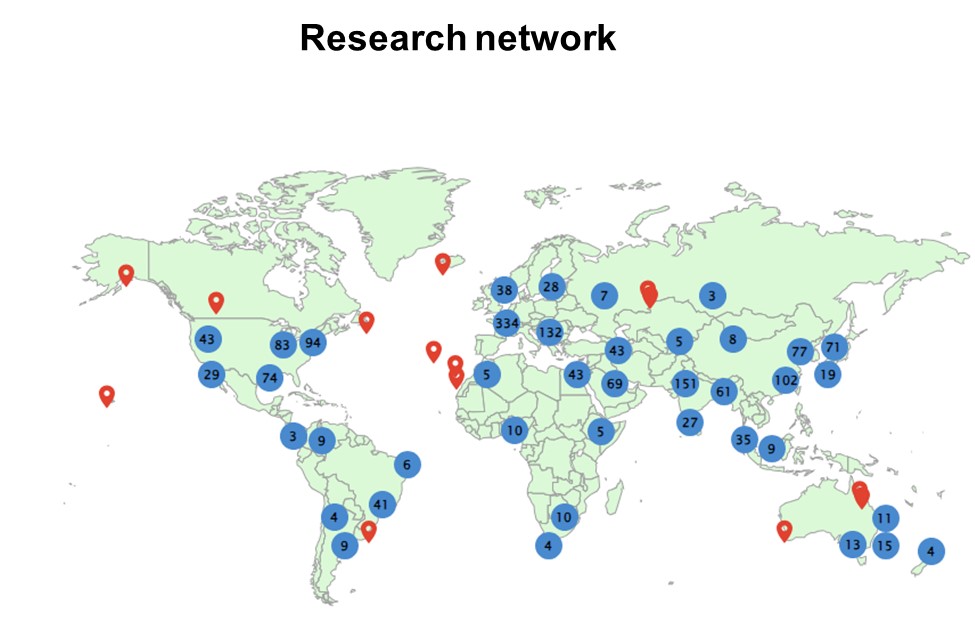
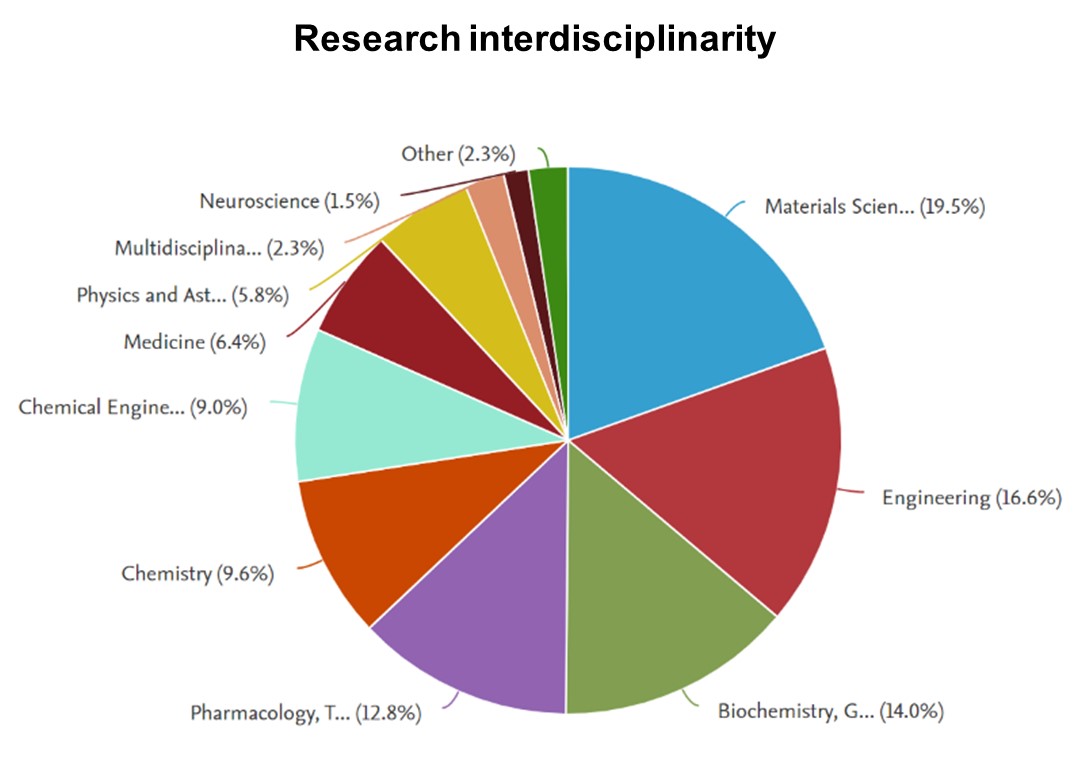
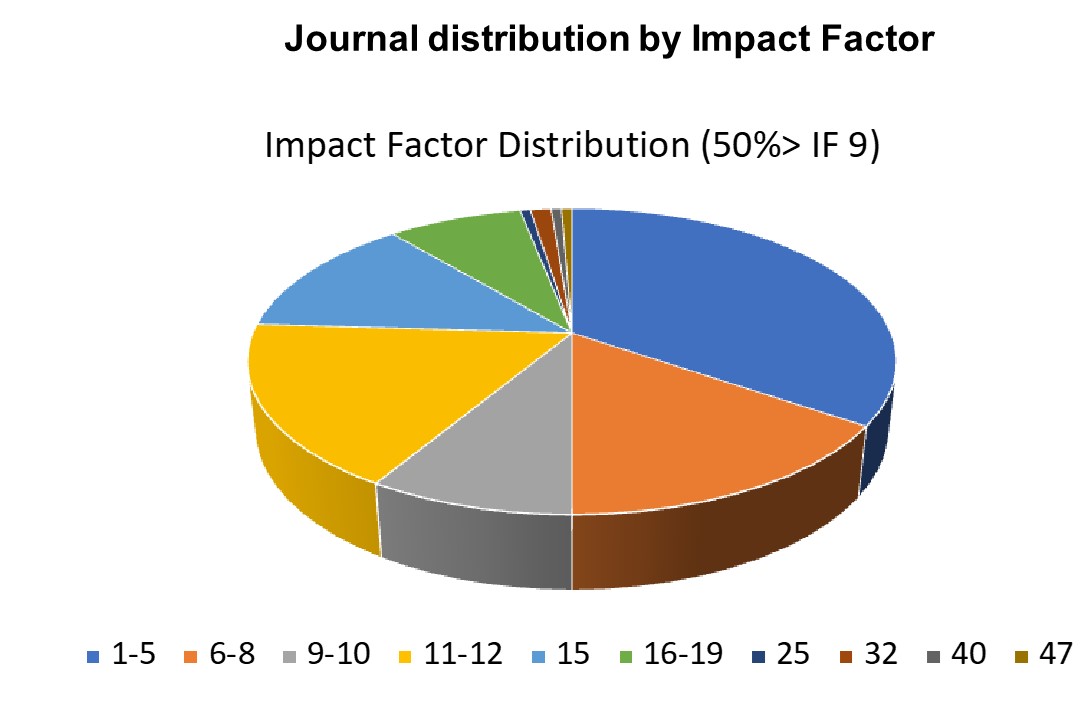
Key Publications
> Selected from 170 papers (accessed 08/24)
 |
 |
 |
 |
Selected Publications
- Rouatbi N, Walters AA, Costa PM, Qin Y, Liam-Or R, Grant V, Pollard SM, Tzu-Wen Wang J and Al-Jamal KT*. RNA Lipid Nanoparticles as Efficient In Vivo CRISPR-Cas9 Gene Editing Tool for Therapeutic Target Validation in Glioblastoma Cancer Stem Cells. J Control Release. 2024. https://doi.org/10.1016/j.jconrel.2024.09.019.
- Liam-Or R, Faruqu FN, Walters A, Han S, Xu L, Wang JT, Oberlaender J, Sanchez-Fueyo A, Lombardi G, Dazzi F, Mailaender V, Al-Jamal KT*. Cellular uptake and in vivo distribution of mesenchymal-stem-cell-derived extracellular vesicles are protein corona dependent Nature Nanotechnology. 2024 Feb 16. doi: 10.1038/s41565-023-01585-y.
- Qin Y, Walters AA, Rouatbi N, Wang JT, Abdel-Bar HM, Al-Jamal KT*. Evaluation of a DoE based approach for comprehensive modelling of the effect of lipid nanoparticle composition on nucleic acid delivery. Biomaterials. 2023 Aug;299:122158. doi: 10.1016/j.
Biomaterials. - Walters AA*, Santacana-Font G, Li J, Routabi N, Qin Y, Claes N, Bals S, Wang JT and Al-Jamal KT*. (2021) Nanoparticle Mediated in Situ Molecular Reprogramming of Immune Checkpoint Interactions for Cancer Immunotherapy. ACS Nano, doi.org/10.1021/acsnano.1c04456
- Abdel-Bar HM, Walters AA, Lim Y, Rouatbi N, Qin Y, Gheidari F, Han S, Osman R, Wang J, Al Jamal KT*. (2021) An “Eat me” Combinatory Nano-formulation for Systemic Immunotherapy of Solid Tumours. Theranostics. doi:10.7150/thno.56936;
Doi:10.7150/thno.56936. - Wang JT, Rodrigo AC, Patterson AK, Hawkins K, Aly MMS, Sun J, Al Jamal KT*, Smith DK*. (2021) Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Advanced Science (Weinh). e2101058. Doi: 10.1002/advs.202101058.
- Xu L, Faruqu FN, Lim YM, Lim KY, Liam-Or R, Walters AA, Lavender P, Fear D, Wells CM, Wang J T-W, Al-Jamal KT*. (2021) Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. Doi.org/10.1016/j.biomaterials.2020.120369120369
- Xu L, Faruqu FN, Liam-Or R, Abu Abed O, Li D, Venner K, Errington RJ, Hummers H, Wang JT and Al Jamal KT. (2020) Design of experiment (DoE)-driven in vitro and in vivo uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. Journal of Extracellular Vesicles. 9(1):1779458. doi.org/10.1080/20013078.2020.1779458.
- Wang JT-W, Klippstein R, Martincic M, Pach E, Feldman R, Sefl M, Michel Y, Sosabowski JK, Kalbac M, Da Ros T, Ménard-Moyon C, Bianco A, Kyriakou I, Emfietzoglou D, Saccavini J-C, Ballesteros B*, Al-Jamal KT* and Tobias G*. (2019) Neutron activated samarium-153 encapsulated single- and multi-walled carbon nanotubes for in vivo imaging and tumour radiotherapy. ACS Nano. doi.org/10.1021/acsnano.9b04898
- Mei KC, Ghazaryan A, Teoh EZ, Summers HD, Li Y, Ballesteros B, Piasecka J, Walters A, Hider RC, Mailänder V, Al-Jamal KT. (2018) Protein-corona-by-design in 2D: a reliable platform to decode bio-nano interactions for the next generation quality-by-design nanomedicines. Advanced Materials. 30, 1802732. doi.org/10.1002/adma.201802732
- Al-Jamal KT, Gherardini L, Bardi G, Nunes A, Guo C, Bussy C, Herrero MA, Bianco A, Prato M, Kostarelos K* and Pizzorusso T*. (2011) Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proceedings of the National Academy of Science. 108:10952-7. doi.org/10.1073/pnas.1100930108 ISSN: 0027-8424.
Patents
- 2024 UK Patent Application No. 2405758.0- New generation PBD payloads, King's College London.
- 2015 United Kingdom Patent Application No. 1520637.8 – Nanocapsules
- 2014 GB1406493.5 - Delivery of NSAIDs via the Nasal Tract to Treat Neurological Disorders (Nabumetone)
- 2010 GB 1000737.5 - Poly-L-Lysine Dendrimers as Systemic Anti-Angiogenic Therapeutics
- 2009 PCT Application No.11066 – Process of Cell Permeabilization Mediated By Nano-Antennas and/or Electromagnetic Fields
Funding
- 2024-2025 Liu Po Shan/Dr Vincent Liu Endowment Fund for MND HK$ 200,00 (PI)
- 2024-2029 Brain Tumour Charity £150,00 (PI)
- 2024-2029 Global STEM Award (PI)
- 2024-2029 Jockey Club STEM Lab Support HKD 10,000,000 (PI)
- 2023-2024 Gut UK £14,000 (Co-I)
- 2022-2025 LifeArc-MND £4.5M (Co-I)
- 2022-2024 Maplethorpe Foundation £140,000 (Sponsor)
- 2021-2022 British Heart Foundation £80,000 (Co-I)
- 2022-2022 Sanofi iAwards Europe program £80,000 (PI)
- 2021-2022 King’s Together Seed Fund £20,000 (PI)
- 2021-2022 King’s Together Seed Fund £20,000 (Co-I)
- 2021-2023 Royal Academy of Engineering £80,000 (Co-I)
- 2022-2026 Schlumberger Foundation Faculty for the Future £150,000 (PI)
- 2022-2025 Brain Research UK £300,000 (PI)
- 2019-2025 Schlumberger Foundation Faculty for the Future £80,000 (PI)
- 2018-2020 Maplethorpe Foundation £160,000 (PI)
- 2017-2019 Maplethorpe Foundation £158,000 (PI)
- 2017-2019 Newton Fund £145,000 (PI)
- 2017-2019 Brain Tumour Charity £98,000 (PI)
- 2017-2019 Marie Sklodowska-Curie Individual Fellowships €183 (PI)
- 2016-2017 KC Wong Foundation £58,000 (PI)
- 2014-2018 Wellcome Trust £250,000 (PI)
- 2012-2016 Worldwide Cancer Research £200,000 (PI)
- 2012-2015 BBSRC New Investigator Award £452,529 (PI)
- 2012-2016 FP7 Marie Curie Initial Training Network £450,000 (UK partner)
- 2011-2012 Royal Society £15,000 (PI)
- 2011-2012 EPSRC Grant Challenge of Nanotechnology in Healthcare £1.5M (Co-I)
- 2005-2007 Maplethorpe Foundation £100,000 (Fellow)





