Professor Aviva S.F. CHOW 周聖峰
Biography
Biography
Professor Aviva Chow received his Bachelor degree of Chemical and Bioproduct Engineering and PhD in Pharmacy from the Hong Kong University of Science and Technology (HKUST) and the Chinese University of Hong Kong (CUHK), respectively. Before his tenure at the University of Hong Kong, he held positions as a Technical Lead and Research Scientist at Jacobson Pharma Corporation Limited. Currently, Professor Chow also serves as the Project Leader and Co-principal investigator at the Advanced Biomedical Instrumentation Centre (ABIC), which was established under the Health@InnoHK Programme. His research interests span a combination of basic, applied, and translational studies on pharmaceutical sciences, with a primary focus on cocrystal engineering and nanoparticle-based drug delivery systems.
Memberships & Editorships
Memberships:- Member of American Association of Pharmaceutical Scientists (AAPS)
- Member of Controlled Release Society (CRS)
Editorship:
- Editorial Board Member, Journal of Pharmaceutical Sciences
- Review Editor, Respiratory Drug Delivery, Frontiers in Drug Delivery
- Review Editor, Solid State Chemistry, Frontiers in Chemistry
Honours and Awards
- Committee member, Committee on Research and Development of Chinese Medicines, Innovation and Technology Commission, HKSAR (2023-present)
- Committee member, Pharmacy and Poisons (Manufacturers Licensing) Committee, Department of Health, HKSAR (2020-present)
- Adjunct Assistant Professor, Department of Chemical and Biological Engineering, HKUST (2018-2020)
- Adjunct Assistant Professor, School of Pharmacy, CUHK (2015-2019)
- Outstanding Reviewer, International Journal of Pharmaceutics, Elsevier (2018)
- Outstanding Reviewer, Chemical Engineering Journal, Elsevier (2017)
- Eli Lilly & Company Travelship Award for AAPS Annual Meeting (2011)
- Pharmaceutical Society of Japan (PSJ) Travelship Award for AAPS Annual Meeting (2010)
- Visiting Research Scholar, Department of Pharmaceutics, College of Pharmacy, University of Minnesota (2009)
Research Interests
Currently, our research group has two specific research foci:
- Cocrystal engineering of pharmaceutical solids
- Design new crystalline forms of drugs with desired pharmaceutical properties
- Development of novel cocrystal-based formulations for pulmonary, intranasal, and transdermal applications
- Mechanistic understanding of cocrystal solution-state stability and its phase transformation
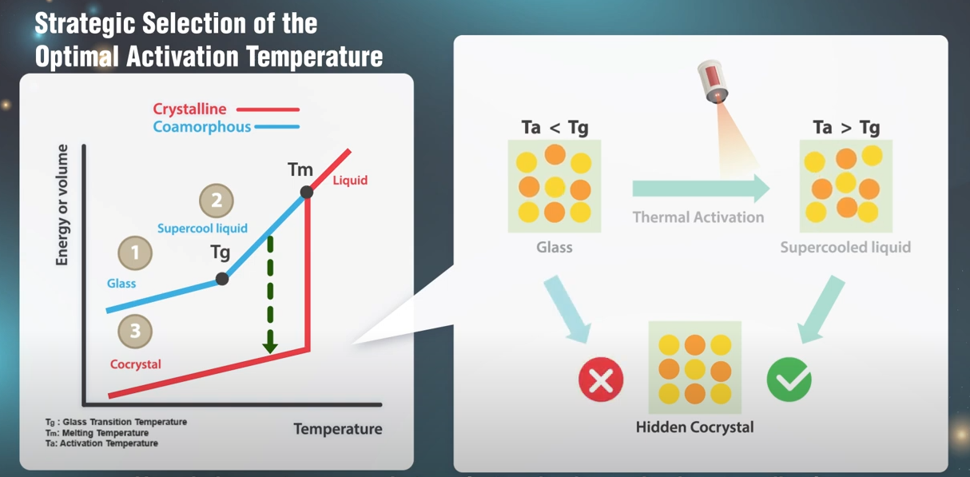
Figure 1: Controlled Thermal Annealing: Role of glass transition and its application for efficient cocrystal screening
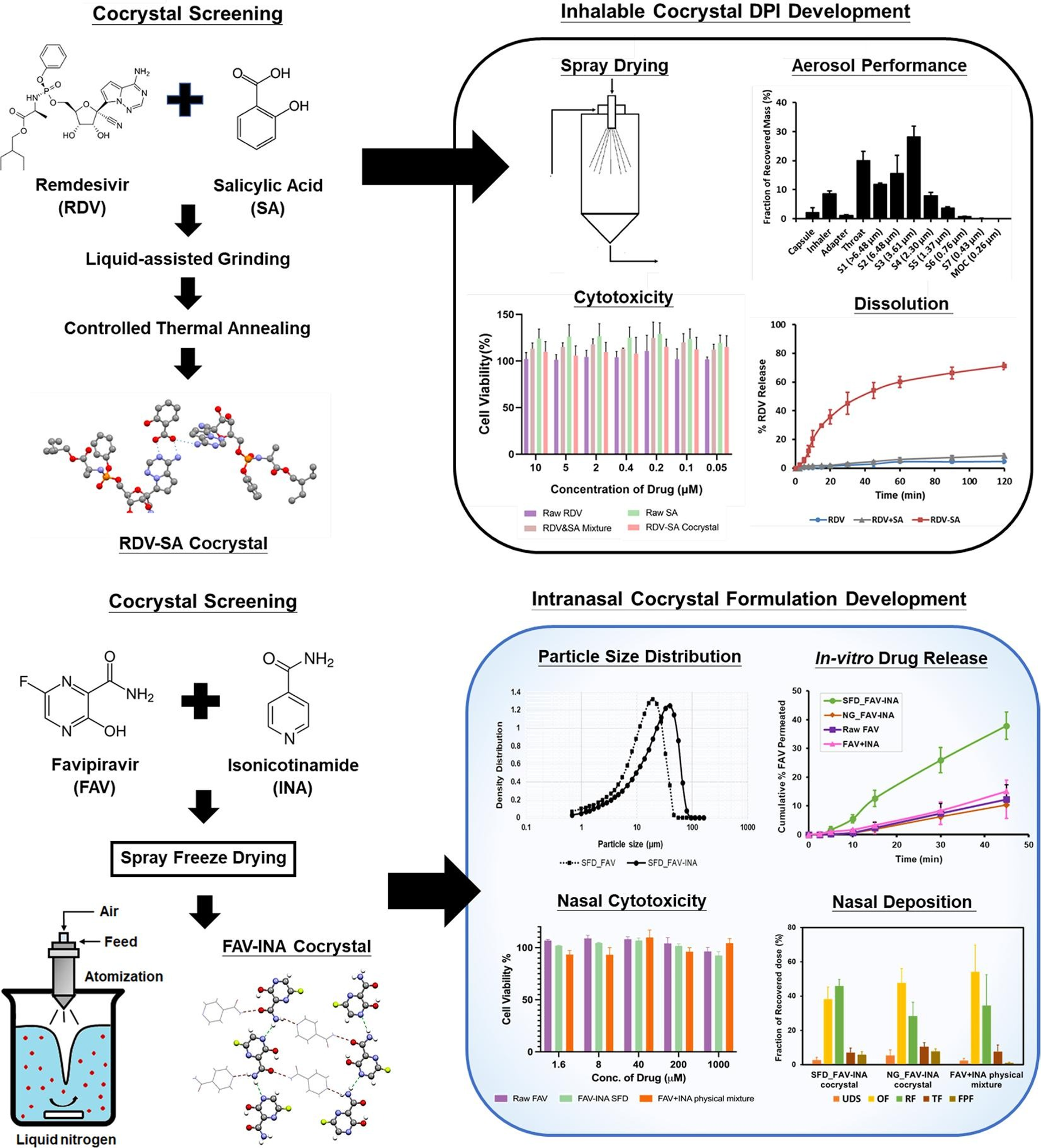
Figure 2: Development of cocrystal-based dry powder formulations for pulmonary and intranasal applications
- Nanoparticle-based drug delivery systems for superior therapeutic outcomes
- Engineering of orally/nasally inhalable drug nanoparticles for optimal pulmonary/nose-to brain delivery
- Understanding the effects of particle size and surface properties on the safety and efficacy of drug nanoparticles
- Design of novel theranostics for management of Alzheimer’s diseases
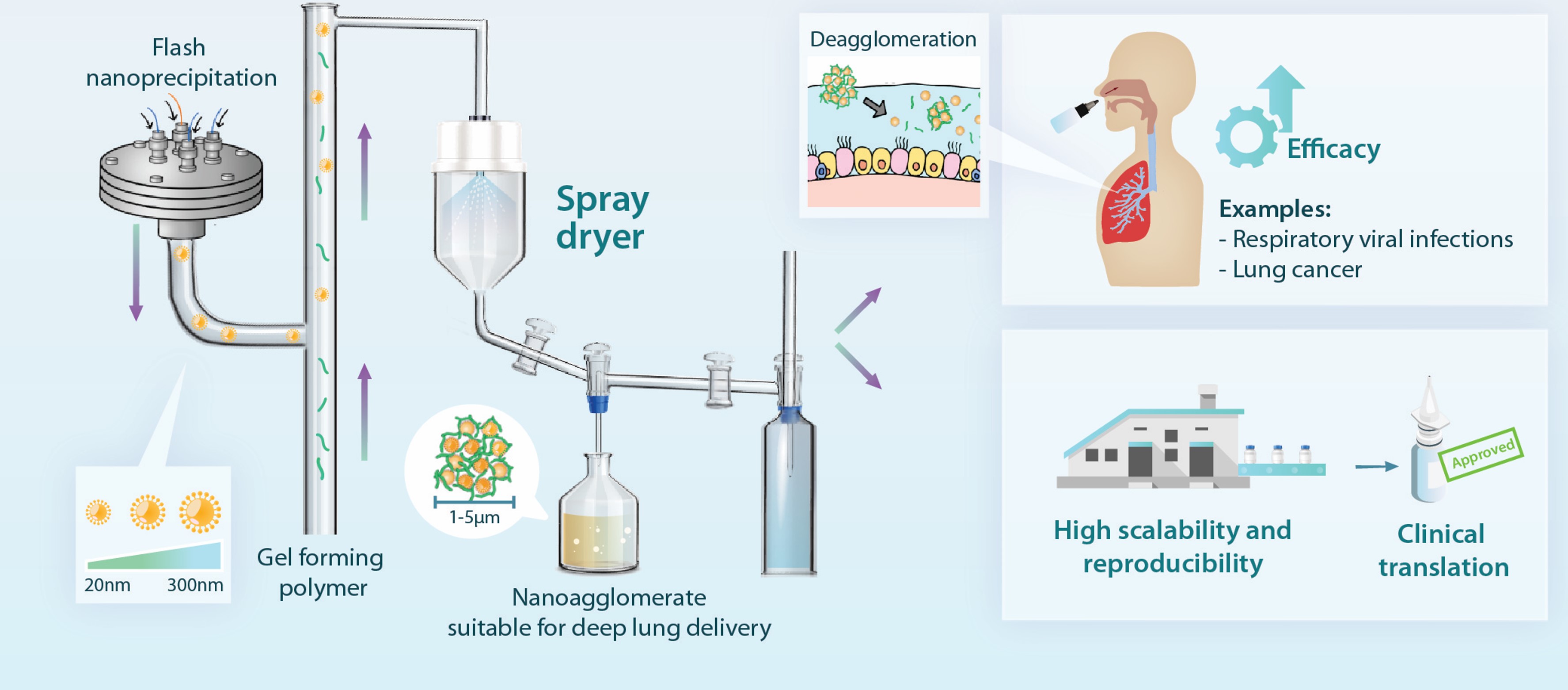
Figure 3: An integrated continuous manufacturing platform in producing dry powders for pulmonary/intranasal drug delivery. It consists of three core technologies, viz., (i) Flash nanoprecipitation; (ii) In-situ thermal gelation; and (iii) Spray drying.
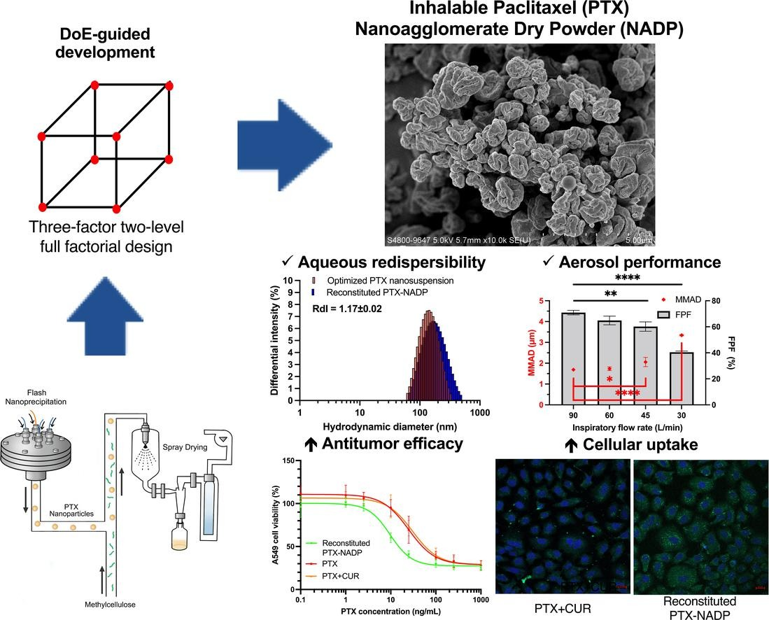
Figure 4: Rational development of inhalable Paclitaxel nanoagglomerate dry powder and its potential application for treatment of non-small cell lung cancer.

Figure 5: Fabrication of different-sized of polymeric drug nanoparticles for optimal drug delivery to organs
Publications
Publication Highlights and Selected Recent Publications:
Cocrystal Engineering:
- S.N. Wong, S. Li, K.H. Low, H.W. Chan, X. Zhang, S. Chow, B. Hui, P.C.Y. Chow, S.F. Chow*. “Development of favipiravir dry powders for intranasal delivery: An integrated cocrystal and particle engineering approach via spray freeze drying”, Int. J. Pharm. (2024), 653, 123896.
- S.N. Wong, K.H. Low, Y.L. Poon, X. Zhang, H.W. Chan, S.F. Chow*. “Synthesis of the first remdesivir cocrystal: design, characterization, and therapeutic potential for pulmonary delivery”, Int. J. Pharm. (2023), 640, 122983.
This research has pioneered the development of the inaugural cocrystal of Remdesivir (an approved drug for COVID-19 treatment), and subsequently reformulated it into an inhalable dry powder for pulmonary delivery. This formulation approach circumvents the metabolic breakdown of Remdesivir into an inactive form, which can occur through oral administration. A US non-provisional patent application has been filed for the cocrystal. - S.N. Wong, J. Weng, I. Ip, R. Chen, R. Lakerveld, R. Telford, N. Blagden, I.J. Scowen, S.F. Chow*. “Rational development of a carrier-free dry powder inhalation formulation for respiratory viral infections via quality by design: a drug-drug cocrystal of favipiravir and theophylline”, Pharmaceutics (2022), 14 (2), 300.
- B. Xuan, Y.C.S. Chen, K.C. Wong, R. Chen, P.S. Lo, R. Lakerveld, H.H.Y. Tong, S.F. Chow*. “Impact of cocrystal solution-state stability on cocrystal dissociation and polymorphic drug recrystallization during dissolution”, International Journal of Pharmaceutics (2021), 610, 121239.
This study studied the phase transformation of cocrystals under various dissolution conditions, providing insights into mitigating the risk of recrystallization of drug polymorphs. It further showcased the potential use of cocrystals in drug polymorph preparation, which is crucial to the evolution of cocrystal-based drug development. - S.N. Wong, S.W.S. Chan, X. Peng, B. Xuan, H.W. Lee, H.H.Y. Tong, S.F. Chow*. “Effects of the glass-forming ability and annealing conditions on cocrystallization behaviors via rapid solvent removal: A case study of voriconazole” Pharmaceutics (2020), 12 (12), 1209.
This reseach demonstrated the importance of a drug’s glass-forming ability and annealing conditions for efficient cocrystal screening. By applying the optimal temperature to surmount high activation energy based on the glass transition behaviours, new cocrystals that are inaccessible by traditional methods can be discovered, thereby expediting new drug development. Our findings have also led to a Silver Medal at the 49th International Exhibition of Inventions in Geneva. - B. Xuan, S.N. Wong, Y. Zhang, J. Weng, H.H.Y. Tong, C. Wang, C.C. Sun, S.F. Chow*. “Extended release of highly water soluble isoniazid attained through cocrystallization with curcumin” Crystal Growth & Design (2020), 20 (3), 1951-1960.
Nanoparticle-based Drug Delivery Systems:
- H.W. Chan, X. Zhang, S. Chow, D.C.L. Lam, S.F. Chow*. “Inhalable paclitaxel nanoagglomerate dry powders for lung cancer chemotherapy: Design of experiments-guided development, characterization and in vitro evaluation”, Int. J. Pharm. (2024), 653, 123877.
- H.W. Chan, H.W. Lee, S. Chow, D.C.L. Lam, S.F. Chow*. “Integrated continuous manufacturing of inhalable remdesivir nanoagglomerate dry powders: Design, optimization and therapeutic potential for respiratory viral infections”, Int. J. Pharm. (2023), 644, 123303.
This study integrated flash nanoprecipitation with our prior in-situ thermal gelation and spray drying technology for a one-step, continuous production of inhalable nanoagglomerate dry powders. The finding derived from this study also led to a US provisional patent application and a Silver Medal at the 49th International Exhibition of Inventions in Geneva. - J. Weng, Z. Shao, H.W. Chan, S.P.Y. Li, J.K.W. Lam, C.K. Tsang, S.F. Chow*. “Mediating bio-fate of polymeric cholecalciferol nanoparticles through rational size control”, Biomater. Adv. (2022), 140, 213074.
This study highlighted the use of Design of Experiment (DoE) in the production of size-tunable nanoparticles with controlled properties. It also delineated the impact of nanoparticles with particle sizes of <200 nm on their bio-fate, facilitating the rational design of drug nanoparticles for targeted therapy. - X. Zhang, L.Y. Chau, H.W. Chan, J. Weng, K.W. Wong, S.F. Chow*, A.H.L. Chow*. "Physical stability and in vivo brain delivery of polymeric ibuprofen nanoparticles fabricated by flash nanoprecipitation", Int. J. Pharm. (2021),120224.
- K.Y. Wan, J. Weng, S.N. Wong, P.C.L. Kwok, S.F. Chow*, A.H.L. Chow*. “Converting nanosuspension into inhalable and redispersible nanoparticles by combined in-situ thermal gelation and spray drying”, Eur. J. Pharm. Biopharm. (2020), 149, 238-247
This research reported a novel technique for fabricating redispersible drug-loaded nanoagglomerates dry powders for deep lung delivery. The findings from this work directly led to a successful grant application from Health and Medical Research Fund from the HKSAR government. - J. Weng, H.H.Y. Tong, S.F. Chow*. “In vitro release study of the polymeric drug nanoparticles: Development and validation of a novel method”, Pharmaceutics (2020), 12, 732
This study developed a novel sample and separate (SS) method for determining the in-vitro drug release profile from polymeric nanoparticles. This method has been widely used by various research groups, receiving > 120 citations and a high field-weight citation impact (FWCI) of 9.71.
For more details, please visit my Scopus and Google Scholar pages:
Scopus: https://www.scopus.com/authid/detail.uri?authorId=54996796900
Google Scholar: https://scholar.google.com/citations?user=I-nvn1oAAAAJ&hl=en&oi=sra
Patents
- S.F. Chow, K.H. Low, S.N. Wong. “Novel cocrystals of dexamethasone”, US non-provisional patent application, 18/307,992, and Chinese Invention Patent application: No. 202310597221.0 (2023).
- S.F. Chow, H.W. Chan, H.W. Lee. “Inhalable agglomerated nanoparticle dry powder pharmaceutical compositions, methods of making and use thereof”, US provisional patent application, 63/498,919 (2023).
- S.F. Chow, K.H. Low, S.N. Wong. “Remdesivir cocrystal, compositions and methods thereof”, US provisional patent application, 63/498,894 (2023).
- A.H.L. Chow, S.F. Chow, X.R. Zhang, K.Y. Wan, K.K. Cheng, L. Baum. “Engineering of polymer-stabilized nanoparticles for drugs with log p values below 6 by controlled antisolvent precipitation”, U.S. Patent, US10076496B2 (2018).
Funding
As PI / Co-PI / Project Leader:
- “Nose-to-brain delivery of combined anti-amyloid-β and anti-tau targeted nanotherapy for Alzheimer’s disease: a pilot study”, Health Medical Research Fund (21223381), Health Bureau (PI, 2024-2026, on-going)
- “Mild magnetic hyperthermia induced by smart amyloid-β peptide targeted nanoparticles: a pilot study for simultaneous diagnosis and therapy of Alzheimer's disease”, Health Medical Research Fund (09201366), Health Bureau (PI, 2022-2025, on-going)
- “Feasibility of next-generation inhalable nanoagglomerated microparticles to improve the therapeutic outcomes of non-small cell lung cancer: A pilot study”, Health Medical Research Fund (08191956), Health Bureau (PI, 2021-2024, on-going)
- “Encapsulating nano-amorphous drugs inside nano-porous media for pulmonary delivery”, Advanced Biomedical Instrumentation Centre Limited, Health@InnoHK Programme, Innovation and Technology Commission (Project Leader, 2021-2025, on-going)
- “腦靶向納米生物醫學技術遞送TERT抑製劑對腦缺血損傷的預防性神經保護”, 協同創新與平台環境建設國際科技合作領域 (2020A0505100045), 廣東省科學技術廳 (Co-PI, HKU-SIRI, 2020-2022, completed)
- “Nebulizing formulation development”, Sponsored Research by a private pharmaceutical company (PI, 2023-2024, on-going)
- “Releasing profile of methylphenidate formulations”, Sponsored Research by a private pharmaceutical company (PI, 2020-2021, completed)
- A feasibility study for developing a spray-dried power formulation containing YQ23”, Sponsored Research by a private pharmaceutical company (PI, 2017-2018, completed)
As Co-I:
- “Inhalable nanoagglomerated pemetrexed and curcumin dry powder for the treatment of lung cancer: a pilot study”, Health Medical Research Fund (21223401), Health Bureau (Co-I, 2024-2026, on-going)
- “Developing a Next-Generation Synbiotic for the Treatment of Metabolic Dysfunction Associated Fatty Liver Disease”, ITSP (Mid-stream, theme-based), Innovation and Technology Commission (Co-I, 2023-2026, on-going)
- “Integrated continuous manufacturing of an active pharmaceutical ingredient for asthma treatment”, Innovation and Technology Support Programme (ITS/137/16), Innovation and Technology Commission (Deputy Project Coordinator and Co-I, 2017-2019, completed)
Other Information
Seeking PhD candidates and Post-doctoral Researchers
Our research group is looking for high-calibers graduate students and post-doctoral researchers interested in the research areas of crystal/cocrystal engineering and nanoparticle-based drug delivery systems.
Full-time postgraduate students are eligible to receive a Postgraduate Scholarship during the normative study period. Outstanding Ph.D. applicants are strongly encouraged to apply for the Hong Kong PhD Fellowship scheme (HKPFS) (https://cerg1.ugc.edu.hk/hkpfs/index.html) and HKU Presidential Scholar Programme (HKU-PS) (https://gradsch.hku.hk/prospective_students/fees_scholarships_and_financial_support/hku_presidential_phd_scholar_programme). Each HKPFS holder/HKU Presidential PhD Scholar will receive a package of up to around HK$433,300 (US$55,560) in the first year, and up to around HK$413,300 (US$52,990) in each of the subsequent years during the normative study period.
Researchers with a PhD degree and strong research track record (e.g., publications, patents, international innovation awards) will be nominated by the PI for the RGC Postdoctoral Fellowship (https://www.ugc.edu.hk/eng/rgc/funding_opport/pdfs/index.html) or Jockey Club Global STEM Post-doctoral Fellowship for Translational Research and Application.
Our current research themes include:
- Cocrystal engineering of pharmaceutical solids
- Development of orally and nasally inhalable nanomedicines for pulmonary and nose-to-brain delivery
- Design of novel nanotheranostics for management of Alzheimer’s disease
- Translational research of Traditional Chinese Medicines beyond oral applications
For further information, please email Professor Aviva Chow with your up-to-date CV (asfchow@hku.hk).
Research Collaborators
- Prof. Calvin Changquan Sun, Department of Pharmaceutics, College of Pharmacy, University of Minnesota, U.S.
- Dr. Jenny Lam, School of Pharmacy, University College London, U.K.
- Dr. Philip Kwok, University of Sydney, Australia
- Prof. Richard Lakerveld, Department of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology, Hong Kong SAR, China
- Prof. Calvin Tsang, Clinical Neuroscience Institute, the First Affiliated Hospital of Jinan University, Guangzhou, China
- Professor David Lam, Department of Medicine, HKU, HKSAR
- Professor Raymond Chang, School of Biomedical Sciences, HKU, HKSAR
Regular Student Consultation Hours
Every Tuesday at 10:00am - 11:00amOffice
2/F, 21 Sassoon Road, Li Ka Shing Faculty of Medicine, Laboratory Block, Faculty of Medicine Building, Hong Kong SAR, China
 |
 |
Professor Aviva Chow and his research team





