Professor Ruby L.C. HOO 何麗莊
Biography
Professor Ruby L.C. Hoo obtained her Bachelor, MPhil and PhD in biological science at The University of Hong Kong. She received her post-doctoral training at division of endocrinology, Department of Medicine at The University of Hong Kong. She took up her post as Research Assistant Professor and Assistant Professor in 2006 and 2017 respectively at Department of Medicine, HKU. She joined the Department of Pharmacology and Pharmacy, HKU, in Feb 2018. She is the principal investigator of research projects funded by Research Grants Council, Innovation and Technology Commission, Human Medical Research Fund, National Natural Science Foundation of China and Guangdong / Shenzhen Basic research fund.
Memberships & Editorships
Memberships:- Co-Investigator, State Key Laboratory of Pharmaceutical Biotechnology
- Member, Research Centre of Heart, Brain, Hormone and Healthy Aging
- Member, HKU Shenzhen Institute of Research and Innovation (HKU-SIRI)
- Ordinary, Hong Kong Society of Endocrinology, Metabolism and Reproduction
- Associate member, Hong Kong Association for the study of obesity
Editorships:
- Frontiers in Endocrinology
- Scientific Reports
Honours and Awards
- Faculty outstanding research output award 2020-2021. Faculty of Medicine, The University of Hong Kong (P.I)
- Faculty outstanding research output award 2019-2020. Faculty of Medicine, The University of Hong Kong (Co-author)
- Excellent presentation award, 2019 International Congress on Obesity and Metabolic Syndrome & Asia-Oceania Conference on Obesity, Korea
- Travel Grant Award, 2019 International Congress on Obesity and Metabolic Syndrome & Asia-Oceania Conference on Obesity, Korea
- Travel Grant Award, 2016 International Congress on Obesity and Metabolic Syndrome in Conjunction with 45th Annual Scientific Meeting of KSSO
- Best oral presentation award, 11th International Symposium on Healthy Aging HKU, 2016
- Best oral presentation award, Medical Research Conference, HKU, 2013
- Best oral presentation award, International Huaxia Congress of Endocrinology, 2012
- Best oral presentation award, Medical Research Conference HKU, 2006
Research Interests
My research interests include the basic and translational research on adipose tissue biology, obesity and its related cardiometabolic diseases. She focuses on deciphering the roles and underlying mechanisms of adipocyte or liver-derived hormones such as adiponectin, fibroblast growth factor-1 (FGF21) and especially adipocyte fatty acid binding protein (A-FABP) in the regulation of energy metabolism and in the pathogenesis of obesity-related cardio-metabolic and inflammatory diseases such as type-2 diabetes, atherosclerosis and non-alcoholic fatty liver disease.
We demonstrated that adiponectin mediates the suppressive effect of the anti-diabetic drug rosiglitazone on plasminogen activator inhibitor-1 production (Hoo RL, 2007; ATVB) and growth hormone could induce the hepatic production of FGF21 via a lipolysis-dependent mechanism in adipocytes (Hoo RL, 2011; J Biol Chem). We also discovered that pharmacological inhibition of A-FABP can significantly alleviate the development of both acute liver injury and non-alcoholic fatty liver disease in mice and A-FABP mediates these diseases by enhancing inflammation in Kupffer cells (Hoo RL, 2013; J Hepatol.). A-FABP is also a mediator of myocardial ischemia/reperfusion injury and diabetes-induced cardiac dysfunction through its suppression on eNOS/NO pathway and induction of superoxide anion production (Hoo RL, 2015; Clin Sci). We showed that A-FABP potentiates toxic lipids-induced endoplasmic reticulum and inflammation in macrophages via inhibition of Janus Kinase 2-dependent autophagy (Hoo RL, 2017; Sci Rep). We also discovered the physiological role of A-FABP in energy metabolism which regulates adaptive thermogenesis through promoting the intracellular conversion of thyroid hormone T4 to T3 in brown adipocytes (Hoo RL & Shu L, 2017 Nat Commun). The study results implicated that global pharmacological inhibition of A-FABP may not be an optimal therapeutic strategy for obesity-related cardiovascular and metabolic diseases due to the potential impairment of adaptive thermogenesis. Recently, our team showed that A-FABP exacerbates ischaemia stroke by inducing the expression of MMP-9 in peripheral macrophages and microglia thus promotes blood-brain barrier disruption (Liao et al., 2020; European Heart Journal). The positively correlation between circulating A-FABP and MMP-9 as well as the infarct volume of the patients was also observed. The data implicating that pharmacological inhibition of A-FABP may be a potential therapeutic strategy for treating the disease. We are now collaborating with Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences for the development of A-FABP monoclonal neutralizing antibody for the treatment of ischemic stroke. In the latest study, our team showed that A-FABP is a key regulator of the onset and progression of liver fibrosis by mediating the crosstalk between liver sinusoidal endothelial cells and hepatic stellate cells (Wu et al., 2021; Advanced Science). This discovery defines A-FABP as a novel therapeutic target for liver fibrosis. Currently, we are exploring the functions of new factors including Syndecan 4 (Sdc4), neutrophil elastase (NE), proteinase 3 (PR3) and the mitochondrial protein Chchd10 that regulate energy metabolism and have potential therapeutic effects on obesity and its related cardiometabolic complications. We are also investigating the potential role of A-FABP in the regulation of bone remodeling.
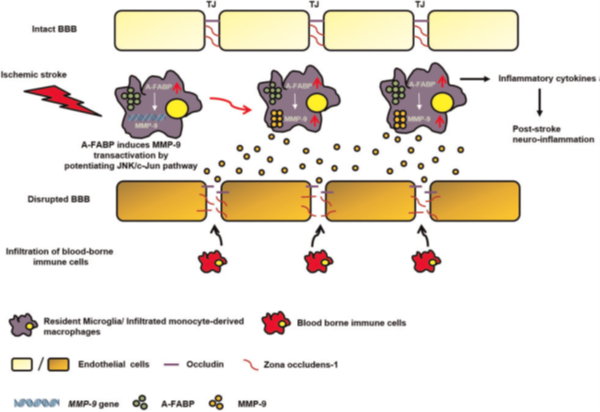
Figure 1: A-FABP mediates BBB disruption and exaggerates ischaemic stroke outcome. In response to ischaemic stroke, the expression of A-FABP is upregulated in peripheral blood monocytes (which further infiltrate in the brain and transform into macrophages) and microglia contributing to the elevation of both circulating and cerebral A-FABP levels. The elevated presence of A-FABP enhances the transactivation of MMP-9 by potentiating JNK activity thus increasing the binding of phosphorylated c-Jun to the AP-1 sites on the MMP-9 promoter. Increased MMP-9 expression and activity further degrade the TJ proteins resulting in BBB disruption which allows the infiltration of blood-borne immune cells thus exacerbates cerebral ischaemic injury. Increased A-FABP in the monocytes-derived macrophages also enhances the expression of pro-inflammatory cytokines contributing to the post-stroke neuro-inflammation. (Liao et al., European Heart Journal, 2020)
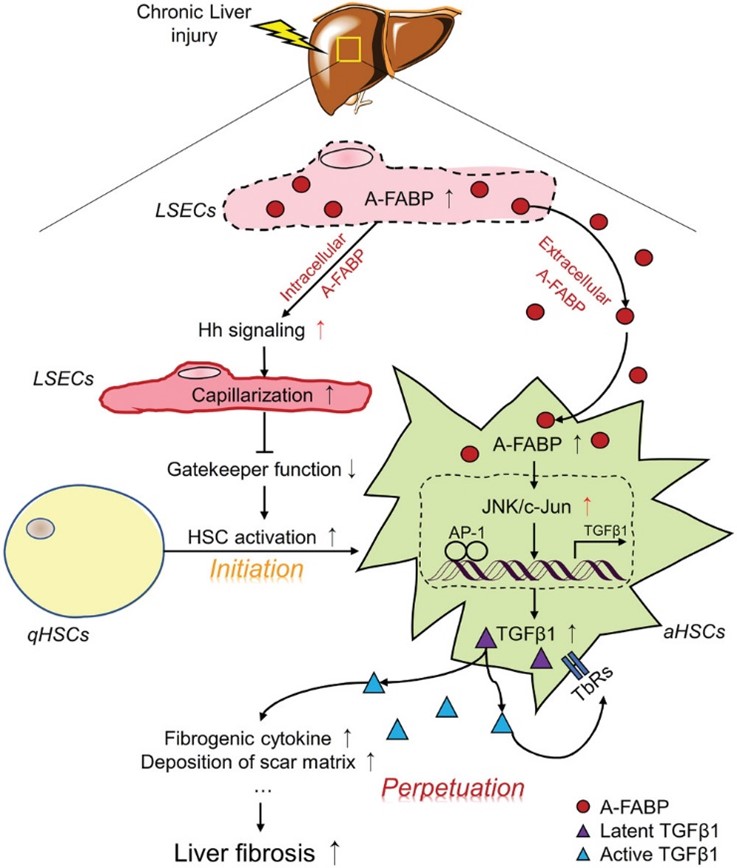
Figure 2: The mechanism whereby A-FABP potentiates liver fibrosis. In response to chronic liver injury, the expression and the subsequent secretion of A-FABP from LSECs are induced. Elevated A-FABP potentiates LSEC capillarization through activating Hh signaling pathway thus initiates HSC activation. On the other hand, LSEC-derived A-FABP acts in a paracrine manner which diffuses into HSCs and stimulates the transactivation of TGFβ1 gene through activating the JNK/c-Jun signaling. Enhanced TGFβ1 perpetuates HSC activation and facilitates the fibrogenic events such as fibrogenic cytokine expression and deposition of scar matrix, therefore exaggerating liver fibrosis. (Wu et al., Advanced Science, 2021)
Publications
Selected Publications
- Wu X, Shu L, Zhang Z, Li J, Zong J, Cheong LY, Ye D, Lam KS, Song E, Wang C, Xu A and Hoo RL* "Adipocyte Fatty Acid Binding Protein Promotes the Onset and Progression of Liver Fibrosis via Mediating the Crosstalk between Liver Sinusoidal Endothelial Cells and Hepatic Stellate Cells" Advanced Science 2021; doi.org/10.1002/advs.202003721 (IF: 17.521)
- Li HL, Wu X, Xu A, Hoo RL*. A-FABP in Metabolic Diseases and the Therapeutic Implications: An Update. International Journal of Molecular Sciences, 2021, 22, 9386. https://doi.org/10.3390/ijms22179386 (IF: 6.208)
- Liao B, Geng L, Zhang F, Shu L, Wei L, Yeung PK, Lam KS, Chung SK, Chang J, Vanhoutte P, Xu A, Wang K*, Hoo RL* Adipocyte fatty acid binding protein exacerbates cerebral ischemia injury by disrupting the blood-brain barrier European Heart Journal, 2020 April; doi.org/10.1093/eurheartj/ehaa207 (IF: 35.855)
- Shu L, Hoo RL*(co-first and co-corresponding author), Wu X, Pan Y, Lee IP, Cheong L, Bornstein SR, Rong X, Guo J, Xu A. Adipocyte fatty acid binding protein mediates adaptive thermogenesis by promoting intracellular activation of thyroid hormones in brown adipocytes. Nat Commun 2017 January doi: 10.1038/ncomms14147 (IF: 17.7)
- Zhou M, Bao Y, Li H, Pan Y, Shu L, Xia Z, Wu D, Lam KS, Vanhoutte PM, Xu A, Jia W, Hoo RL*. Deficiency of adipocyte fatty-acid-binding protein alleviates myocardial ischaemia/reperfusion injury and diabetes-induced cardiac dysfunction. Clin Sci. 2015 Oct;129(7):547-59. doi: 10.1042/CS20150073. (IF: 6.876)
- Hoo RL, Lee IP, Zhou M, Wong JY, Hui X, Xu A, Lam KS. Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non-alcoholic steatohepatitis in mice. J Hepatol. 2013 Feb;58(2):358-64. doi: 10.1016/j.jhep.2012.10.022. (IF: 25.083)
- Chen W, Hoo RL (co-first author), Konishi M, Itoh N, Lee PC, Ye HY, Lam KS, Xu A. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011 Oct 7;286(40):34559-66. doi: 10.1074/jbc.M111.285965. (IF: 5.157)
- Hoo RL, Wong JY, Qiao C, Xu A, Xu H, Lam KS. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr Metab (Lond). 2010 Aug 24;7:67. doi: 10.1186/1743-7075-7-67. (IF: 3.93)
- Hoo RL, Yeung DC, Lam KS, Xu A. Inflammatory biomarkers associated with obesity and insulin resistance: a focus on lipocalin-2 and adipocyte fatty acid-binding protein. Expert Rev Endocrinol Metab. 2008 Jan;3(1):29-41. doi: 10.1586/17446651.3.1.29. (IF: 3.55)
- Hoo RL, Chow WS, Yau MH, Xu A, Tso AW, Tse HF, Fong CH, Tam S, Chan L, Lam KS. Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor-1 production. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2777-82.(IF: 10.514)
- Ye H, Zhang HJ, Xu A, Hoo RL*. Resistin production from adipose tissue is decreased in db/db obese mice, and is reversed by rosiglitazone. PLoS One. 2013 Jun 12;8(6):e65543.doi: 10.1371/journal.pone.0065543. (IF:3.75)
- Hoo RL* (first and corresponding author), Shu L, Cheng KK, Wu X, Liao B, Wu D, Zhou Z, A Xu. Adipocyte Fatty Acid Binding Protein Potentiates Toxic lipids-Induced Endoplasmic Reticulum Stress in Macrophages via Inhibition of Janus Kinase 2-dependent Autophagy. Sci Rep 2017 January doi: 10.1038/srep40657 (IF:4.996)
- Wong TL, Ng KY, Tan KV, Chan LH, Zhou L, Che N, Hoo RL, Lee TK, Richard S, Lo CM, Man K, Khong PL, Ma S. CRAF methylation by PRMT6 regulates aerobic glycolysis driven hepatocarcinogenesis via ERK-dependent PKM2 nuclear relocalization and activation. Hepatology 2020 Apr;71(4):1279-1296. doi: 10.1002/hep.30923 (IF: 30.083)
- Lee TH, Cheng KK, Hoo RL, Siu PM, Yau SY. The novel Perspectives of adipokines on brain health. Int J Mol Sci. 2019 Nov 11;20(22). pii: E5638. doi: 10.3390/ijms20225638. (IF: 6.208)
- Pan Y, Hui X, Hoo RL, Ye D, Chan CYC, Feng T, Wang Y, Lam KSL, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019 Feb 1;129(2):834-849. doi: 10.1172/JCI123069. (IF: 19.456)
- Ng RC, Cheng OY, Kwan JS, Ho PW, Cheng KK, Yeung PK, Zhou LL, Hoo RL, Chung SK; Xu A, Lam KS, Chan KH. Chronic adiponectin deficiency leads to Alzheimer's disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Molecular Neurodegeneration 2016 Nov 25;11(1):71. (IF: 18.879)
- Kwok KH, Cheng KK, Hoo RL, Ye D, Xu A Lam KS. Adipose-Specific Inactivation of JNK Alleviates Atherosclerosis in ApoE-deficient Mice Clin Sci. 2016 Nov 1;130(22):2087-2100. (IF:5.629)
- Li X, Cheng KK, Wang B, Liu Z, Hallenborg P, Hoo RL, Lam KS, Ikeda Y, Gao X, Xu A. The MDM2-p53-pyruvate carboxylase axis couples mitochondrial metabolism to glucose-stimulated insulin secretion in pancreatic β cells. Nature Communications. 2016 June doi:10.1038/ncomms11740. (IF:17.7)
- Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15810-5. doi: 10.1073/pnas.1415219111. (IF:9.423)
- Lin Z, Tian H, Lam KS, Lin S, Hoo RL, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013 May 7;17(5):779-89. doi: 10.1016/j.cmet.2013.04.005. (IF: 17.565)
Patents
Hoo RL, Xu A, Chang J, Liao, B. Yang S, Li, S. Application of monoclonal A-FABP antibody for the treatment of ischemic stroke (一種A-FABP 中和單克隆抗體及其製備方法和用途); Chinease patent filing no: IP01220
Funding
Grant awarded as PI
- Adipocyte fatty acid binding protein (A-FABP) as a potential therapeutic for osteoporosis (HMRF; 09201826; HKD$1,497,000, 07/2022-06/2025)
- 線粒體蛋白chchd10在肥胖相關糖尿病性心肌病中的作用及機制研究. (廣東省自然科學基金面上項目); 2022A1515010533, RMB 100,000; 01/2022-12/2024)
- Syndecan-4 (Sdc4) as a key regulator of white adipose tissue browning for energy expenditure (GRF; 17101819; HK$1,113,341 01/01/2020-30/06/2023)
- Development and Preclinical Evaluation of a A-FABP Neutralizing Monoclonal Antibody for Ischemic Stroke Treatment. 基於A-FABP中和單克隆抗體的缺血性腦卒中治療藥物的研製與臨床前評估. (ITC-FASII; GHP/079/18SZ; HK $1,327,458; 01/09/2019-30/06/2022)
- Mitochondrial protein Coiled-Coil-Helix-Coiled-coil-Helix Domain 10 (Chchd10) as a novel factor for the treatment of obesity and its related diseases. (HMRF; 06172646; HKD$ 982,396 1/6/2019-15/5/2022)
- Neutrophil serine proteases as the endogenous suppressors of white adipose tissue browning and adaptive thermogenesis (RGC GRF; 17105318; HKD$ 775,343; 01/2019-06/2022)
- 脂肪細胞型脂肪酸結合蛋白(A-FABP)在腦缺血性中風中的作用機制研究 (Natural Science Foundation of China; 81770885; RMB$560,000 1/2018-12/2021)
- 脂肪細胞脂肪酸結合蛋白(A-FABP)調控局部脂肪組織重塑和擴張的作用及機制研究 (Shenzhen Basic Research Grant; 201605303000678; RMB $300,000 1/1/2017-31/12/2019)
- Adipocyte fatty acid binding protein (A-FABP) is a pathological mediator of obesity-related renal dysfunction. (HMRF; 03143846; HKD $1,035,108; 1/6/2016-31/5/2018)
- Adipocyte fatty acid binding protein (A-FABP) is a potential therapeutic target of both alcoholic and non-alcoholic steatohepatitis. (HMRF; 02131906; HKD $ 904736; 1/4/2015-30/9-2017)
- 脂肪細胞型脂肪酸結合蛋白在產熱效應及白色脂肪棕色化中的作用及機制研究 (Shenzhen Basic Research grant; JCYJ20140903112959965; RMB$300,000: 1/1/2015- 31/12/2016)
- Adipocyte fatty acid binding protein (A-FABP) as a potential mediator of cardiac dysfunction and heart failure related to diabetes and obesity. (RGC GRF; HKU767913, HKD $ 736,128; 1/2014-12/2016)
- 脂肪細胞型脂肪酸結合蛋白(A-FABP)在毒性脂質誘導的巨噬細胞炎症反應中的作用機制研 (NSFC; 81270862, HKD $879200; 1/2013-12/2016)
- Interplay between adipocyte fatty acid binding protein and endoplasmic reticulum stress in toxic lipids-induced inflammation in macrophages. (RGC GRF; HKU766812, HKD $750,000; 1/2013-12/2014)
Grant awarded as Co-PI
- Discovery and Development of Innovative Antibody Drugs for Metabolic and Cardiovascular Diseases (CAS - Croucher Funding Scheme for Joint Laboratories, HKD 3750000; 03/2021- 02/2024)
- Identification of novel susceptibility loci for diabetic retinopathy in Chinese patients with type 2 diabetes: an Asian Screening Array analysis (RGC GRF:17118119, HKD $1115228; 01/2020-12/2021)
- Institute of Metabolic medicine (RGC-AoE_M707/18; HKD $ 77803000; 05/2019-04/2027
- Conversion of white into brown adipocytes as a therapeutic strategy for obesity-related metabolic and vascular complications (CRF; C7037-17; HKD$ 7430000; 06/2018 – 06/2021)
- The oncogene MDM2 as a new mediator of obesity-induced nonalcoholic fatty liver disease (HMRF; 05161286; HKD$1191000; 04/2018-03/2020)
- TRIF as a novel regulator of lipid droplet enlargement in visceral adipocytes (RGC; 17104721; HKD$ 1125732; 01/09/2021-31/8/2024)
- PPM1K restricts macrophage inflammation by integrating BCAA catabolism and mitochondrial metabolism in obesity (RGC; 15101221, HKD$ 1174432; 01/01/2022-31/12/2024)
Other Information
Current Team Members
- Ms Amber Xiaoping WU (Research Assistant Professor)
- Ms Yuan Lufengzi (PhD candidate)
- Ms Zong Jiuyu (PhD candidate)
- Ms. Viola Zixuan ZHANG (PhD candidate)
- Ms. Jiang Mengxue (PhD candidate)
- Ms Ping Zhihui (Research Assistant)
Students Awards
- Dr. Lingling SHU: First place in the best abstract in basic science & translational (oral presentation) at the 24th Medical Research Conference”, The University of Hong Kong, Jan 2019. Title: FABP4 mediates autoimmune diabetes through activation on macrophage and tissue resident memory T cells.
- Ms Amber Xiaoping WU: Second place in “Best Abstract in Basic Science & Translational (Oral) at the 23rd Annual Medical Research Conference, The University of Hong Kong, Janurary 2017. Title: The role of adipocyte fatty acid binding protein in the pathogenesis of liver fibrosis.
- Dr. Lingling SHU: Young Investigator Award in 10th International Symposium on Health and Aging, Hong Kong, March 2015.
- Dr. Lingling SHU: First Runner-up in Best Oral Presentation at 13th Annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Diseases in Los Angeles, America, Nov 2015.






